
Fluid handling is a sub-area of the pharmaceutical industry. This segment includes solutions for the transport of liquid drugs – from cough syrup to complex active pharmaceutical ingredients (APIs) such as vaccines or specific medications for cancer treatment. Products in the fluid-handling section are used in the biotechnology, pharmaceutical and medical markets, all highly regulated environments.
Quality Management
In the pharmaceutical industry, a comprehensive and expertly implemented quality management system is indispensable. Standardised processes contribute to consistently high product quality. These processes are regularly checked and continuously improved.
Custom Development
You determine the ideal solution for your needs. Even multi-year development periods do not pose a problem. Ultimately, only a perfect outcome for the customer matters. You receive everything from a single source: From development work on your product to specific formulations, through to production and follow-up service. You will benefit from having one contact for all of your needs.
Providing Biopharmaceutical Industries with Innovative Products and Solutions
Fluid Biosolutions represents a growing number of global manufacturers that deals with single-use technology, silicone tubing, pharmaceutical pumps, and equipment supplies. Our commitment to our clients enables us to be hands-on in guaranteeing you get the best product suited to your applications. From selecting the most appropriate tubing right through to custom designing complex single-use assemblies, we offer local expertise supported by world-class products.
Supplier of Key Products and Single-Use Solutions for Critical Fluid Management
Since our establishment in 2015, we have been a trusted supplier to the Australian pharmaceutical industry of products and customised single-use solutions for critical aseptic fluid processes. With over 25 years of experience in the pharmaceutical and life science industries, we pride ourselves on the personalised support we can offer our customers, backed up by the very highest quality products and services.
Fluid Biosolutions are dedicated to the supply and support of a range of high-quality products for use in the manufacture of sterile pharmaceutical drugs. With a focus on fluid handling, we work closely with our clients to ensure the best solutions for their applications. This can often include the design of custom products, particularly with the increasing trend towards single-use technology. And the individual nature of a client’s upstream or downstream processes.
At Fluid Biosolutions we value quality in the products we supply, and the customer support we offer our clients. So, if you are looking for the best tubing for your application or are designing a complex single-use manufacturing process, we are here to support you.
With the growth of single-use manufacturing in the pharmaceutical and bio-processing industries, came the need to create products to meet the market demand. Biopharmaceutical companies then have the challenge of designing their manufacturing processes around these standard products.
The philosophy at Fluid Biosolutions is to work collaboratively with our customers to design application-specific solutions that will meet their processing requirements. Such can include the design of single-use tubing manifolds, which can incorporate a wide range of additional components like filters, sensors, bags, sterile connectors, sampling bottles and more.
If you have an application that would suit a customised design or have a current process that you would like to optimise – contact us, and we can work with you to create a solution that will cater to your specific requirements.
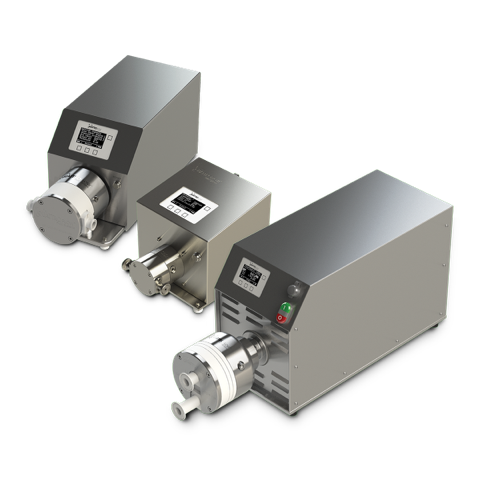
Quattroflow™ develops and manufactures, in close cooperation with our customers, quaternary (four-piston) diaphragm pumps specifically designed for critical applications in the biopharma, and food and beverage industries.
How Quattroflow™ Works?
The Quattroflow pump’s method of operation allows it to gently, safely and securely convey aqueous solutions and biological products that are shear sensitive. The four-piston design does not include a mechanical shaft seal or wetted rotating parts, ensuring total product containment without abrasion. Additionally, the four-piston pumping principle enables risk-free dry running, low pulsation, self-priming, and minimal particle generation. Quattroflow pumps are available with cleanable multiple-use stainless steel or disposable single-use plastic pump chambers.
Truly unique in that their method of operation, Quattroflow Quaternary Diaphragm Pumps mimic the human heart, which is the perfect model for the safe, reliable and efficient transfer of biological materials. With an operation that offers low friction, low shear and low pulsation, Quattroflow pumps are ideal for critical applications within biopharma manufacturing. These include Tangential Flow Filtration (TFF), Chromatography (RFC), centrifuge feed, sterile virus filtration, purification and inline dilution. Quattroflow pumps can also be outfitted with a single-use liquid chamber, which enables them to meet strict speed-to-market manufacturing demands.
Quattroflow™’s Latest Innovation: Q-Control Integrated Pump Controller
Quattroflow™, part of PSG®, a Dover company and a leading brand of positive displacement quaternary diaphragm pumps, is pleased to announce the availability of its new Q-Control Integrated Pump Controller, which is designed to interface with a variety of flow and pressure sensors to provide users with automated control over their pump operations.
Available for Quattroflow multiple- and single-use quaternary diaphragm pump models QF30, QF150, QF1200, QF2500 and QF4400, the Q-Control eliminates the need for an external PLC thanks to a variety of built-in smart control functions, including an autotune function that automatically finds optimal PID parameters and a dispensing function that allows the pump to automatically fill defined volumes of liquid. The system also allows users to configure alarms to stop the pump and features an RS485 Modbus communication port, trend data, alarm logging and remote operation.
Quattroflow pumps primarily serve biopharma applications that require gentle displacement, reliability, product safety, purity and accuracy. The pumping principle enables risk-free dry running, low pulsation, self-priming, and minimal particle generation. With single- and multiple-use models available, Quattroflow has a pump to fit every application need.
About Quattroflow™:
Quattroflow™ is a leading brand of quaternary (four-piston) diaphragm pumps that primarily serve industries such as pharmaceutical and biopharmaceutical that require gentle displacement, reliability, product safety, purity and cleanability. Headquartered in Duisburg, Germany, Quattroflow is part of PSG®, a Dover company. For more information on Quattroflow and its complete family of four-piston pumps, please visit quattroflow.com.
PSG® is a global pump solution expert and leading manufacturer of pumps, systems and related flow-control technology for the safe and efficient transfer of critical and valuable fluids and materials. Headquartered in Oakbrook Terrace, IL, USA, PSG is comprised of several world-class brands, including Abaque™, All-Flo, Almatec®, Blackmer®, Ebsray®, em-tec, EnviroGear®, Griswold®, Hydro Systems, Mouvex®, Neptune™, Quattroflow™, RedScrew™ and Wilden®. PSG products are manufactured on three continents – North America, Europe and Asia – in state-of-the-art facilities that practice lean manufacturing and are ISO-certified.
Article source:

Our open-architecture approach allows our customers to customize assemblies using our vast library of components. Vertically integrated components such as Pure-Fit® SIB connectors, BarbLock® ultra-secure retainers, and single-use bioprocess bags, coupled with our industry-proven silicone tubing, SPT-60L, can help to achieve your filling process requirements.
Testing to regulatory standards ensures the cleanliness, biocompatibility, and sterility of assemblies as evidenced through the quality documentation provided with your products. Our seamless flow path overmoulding technology and range of tubing products support critical fluid path requirements for accuracy of volume delivery, reduced pulsation during filling, and minimized hold-up. Leverage our relationships with filling needle and DPTE transfer bag manufacturers, which among those include the Getinge DPTE-BetaBag® product, for a complete filling assembly solution.
Final Fill Custom Assembly Components
Line Identification
Line set identification via number tags or colour coding is available on assemblies to simplify operator installation and setup of the filling line.
Multi-Port Adaptor
The multi-port adaptor simplifies the final fill assembly design by consolidating flow management into a single manifold versus multiple leg designs. This reduces the ‘spaghetti’ of single-use designs allowing for easier deployment of tubing sets.
Bioprocess Bag
Single-use bio process bags are constructed of a multilayer film optimized for bioprocessing applications, including an LDPE product contact layer, an EVOH gas barrier, and a nylon outline. The film materials are consistent throughout the product range and are free of animal-derived components with supporting BPOG extractable information. Offering outstanding biocompatibility, chemical compatibility, barrier and strength properties, Saint-Gobain bioprocess bags meet the most demanding bioprocess applications.
Our testing successfully identified Saint-Gobain’s 5-layer film bioprocess bags as a suitable material for use in final fill applications with VHP exposure.
Port Disk Adaptor
Our patented port disk adaptor provides a novel solution for fluid transfer applications with beta bags. The over-moulded adapter eliminates fluid path dead zones introduced by barbed fittings and allows for smaller ID tubing to be used with the bag port (no step-up / down required). Removing several mechanical connections on the assembly using the port disk adapter reduces potential leak points for tubing adaption to the bag port. The port disk adapter is also available on custom bioprocess bag configurations.
DPTE-BetaBag®
Getinge single-use DPTE-BetaBag® is designed for fast contamination-free transfer. The flexible bag docks onto a DPTE® Alpha port for a safe leak-tight connection to a sterile zone.
Overmolding
As a pioneer in overmolding technology, Saint-Gobain utilizes overmolding in pump tubing segments to give hours of continuous, leak-free operation. Our industry-recognized SPT-60L silicone tubing is long life and provides consistent delivery over the filling lifecycle with minimal spallation.
Additional over-moulded connections are available (i.e. reducers, manifolds, tees, etc.) and can be customized to your specific equipment or configuration in Sani-Tech® silicone or C-Flex® TPE resins.
Needle Protection
Unique design for protection of needles during packaging/shipment facilitates extraction of line sets from BetaBag and improves handling and deployment of line sets in the isolator. Needles are protected by the puncture-resistant, polyurethane sleeves or can be secured in a moulded polypropylene case (in groups of two).
Needles
Needles can be sourced from several needle suppliers (stainless steel and polymer) to support specific equipment and process needs.
Article Source:
https://www.biopharm.saint-gobain.com/product-category/final-fill-custom-assemblies