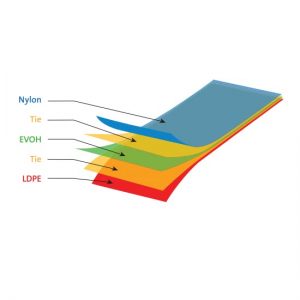
Description
Saint-Gobain Life Sciences is a global supplier of 50mL to 3,000L single-use bioprocess bags for the biopharmaceutical industry. Produced at multiple manufacturing facilities, their single-use bags are constructed of a multilayer film optimised for bioprocessing applications.
The film materials are consistent throughout the product range and free of animal derived components. Offering outstanding biocompatibility, chemical compatibility, barrier and strength properties, Saint-Gobain films meet the most demanding bioprocess applications.
Saint-Gobain BioProcess Systems are manufactured following cGMP that is QSR and ISO 9001 : 2008 compliant.
Bags are manufactured and assembled at our Plymouth, MN facility that operates a certified ISO Class 7 cleanroom. We are a leading global supplier of 50 ml to 3,000 L single-use bags for the biopharmaceutical industry.